SPECTRALLY-RESOLVED PLANAR LASER-INDUCED FLUORESCENCE (SR-PLIF)
|
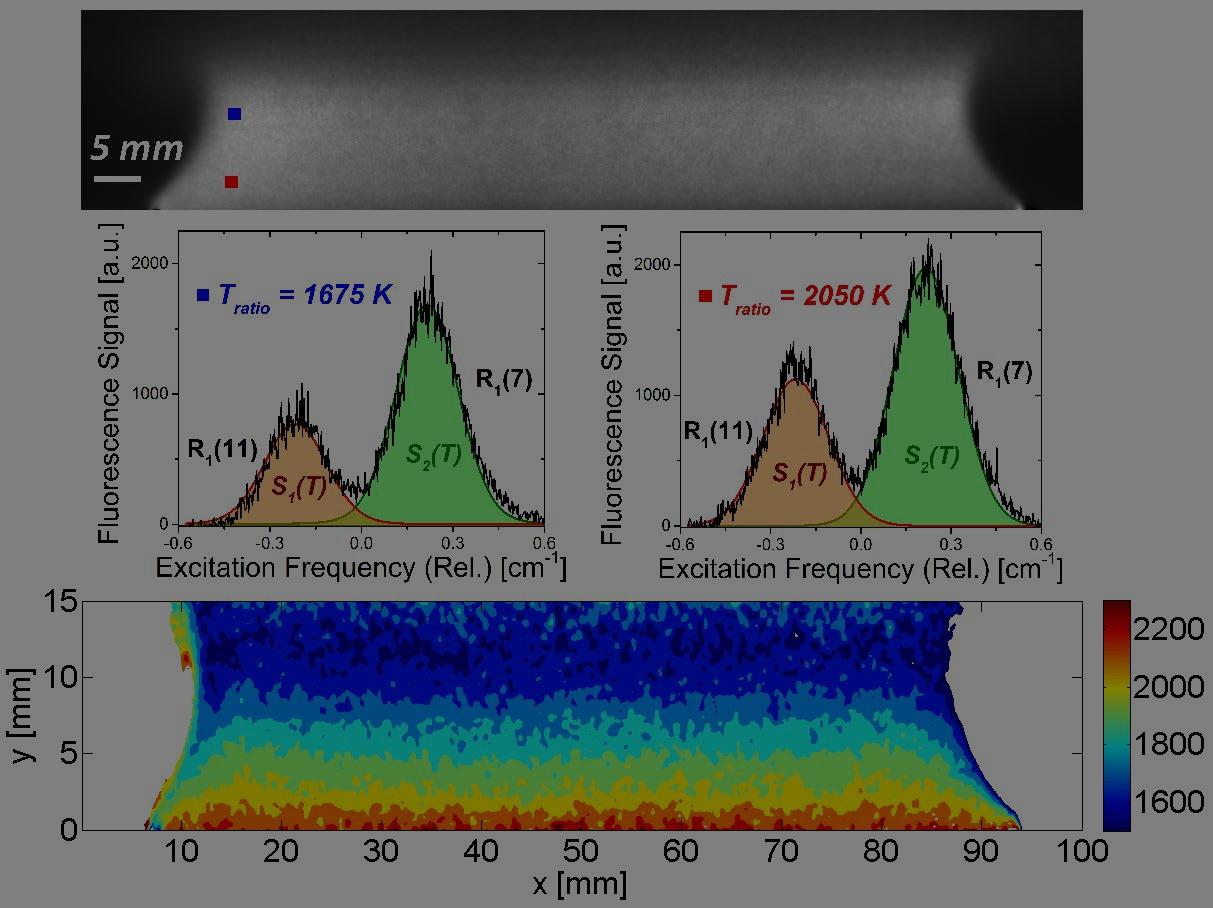
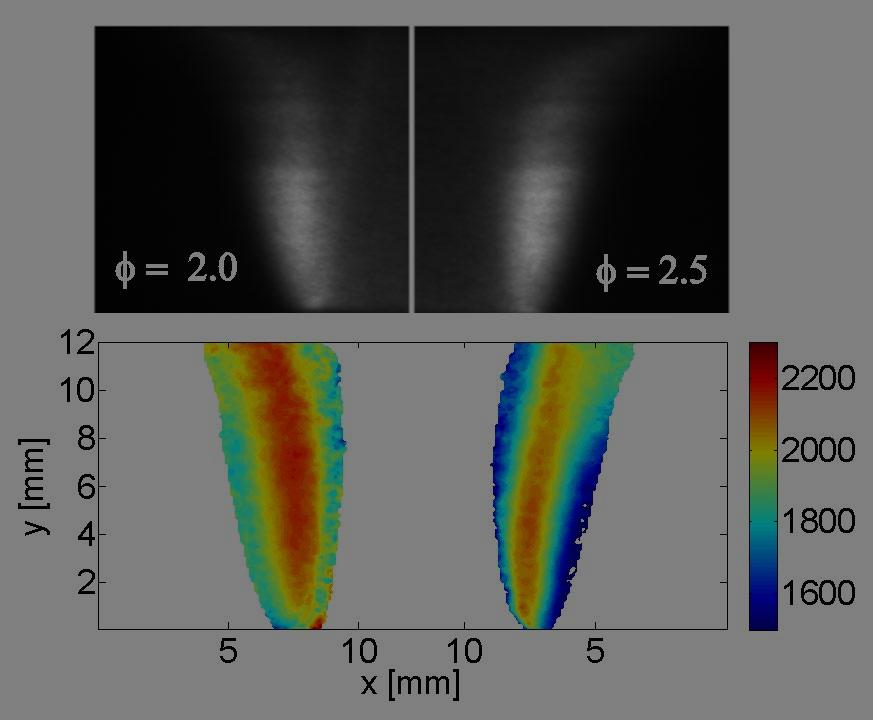
It has been a “holy grail” quest in experimental studies of compressible and reacting flows to find a strategy for simultaneous quantification of temperature, pressure, velocity and species composition in a spatially- and temporally-resolved manner (i.e. "to measure everything, everywhere, at all time"). The current research aims to tackle this extremely challenging problem by introducing a novel method, namely Spectrally-Resolved Planar Laser-Induced Fluorescence (SR-PLIF). This method utilizes high-power, rapid-tuning, narrow-linewidth CW lasers to access the spectral lineshape of selected molecular transitions in the entire measurement plane, which enables determination of multiple key quantities in complex flow fields (e.g. temperature from line ratio, pressure from line width, velocity from Doppler shift, and concentration from fluorescence intensity). Combining the 2-D simultaneous measurement capability with precision lineshape-resolved spectroscopy, this method represents a game-changing extension to conventional PLIF techniques. A preliminary demonstration of this method for 2-D temperature measurement is shown in Fig. 1. This demonstration was performed over a burner-stabilized stoichiometric CH4-air flat flame, where the R1(11) ∕ R1(7) line pair of the OH A2Σ−X2Π (0,0) rovibronic band was probed. The top panel shows a representative frame of the SR-PLIF signal; the middle panel shows the fluorescence spectra at two different points above the flame, where temperatures at these points were inferred from the intensity ratio between the integrated fluorescence signals of the two OH transitions; the bottom panel shows the inferred temperature field of the whole measurement plane. Note that the measurement was done through a single wavelength scan within 0.25 second, and the uncertainty in the measured temperatures was less than 2%. Besides, no calibration was needed, which overcame a substantial limitation in conventional PLIF thermometry. Fig. 2 shows another set of examples where measurements were conducted in cylindrical diffusion flames of CH4. The current research on the SR-PLIF methodology is still at the very early stage, and much efforts are still needed to realize its full potential. Improvement of measurement accuracy and dynamic range, adaptation to harsh environments, and extension to measurements of other physical properties and tracer molecules, are currently being investigated. Other aspects of on-going research include extending the method to high speed for studies of time-varying and transient phenomena, as well as to 3-D measurements of complex flow or flame structures, etc. This line of research is pursued through collaboration with Stanford University and Purdue University. |
|
PRECISION LASER DIAGNOSTIC FOR OH RADICAL
|
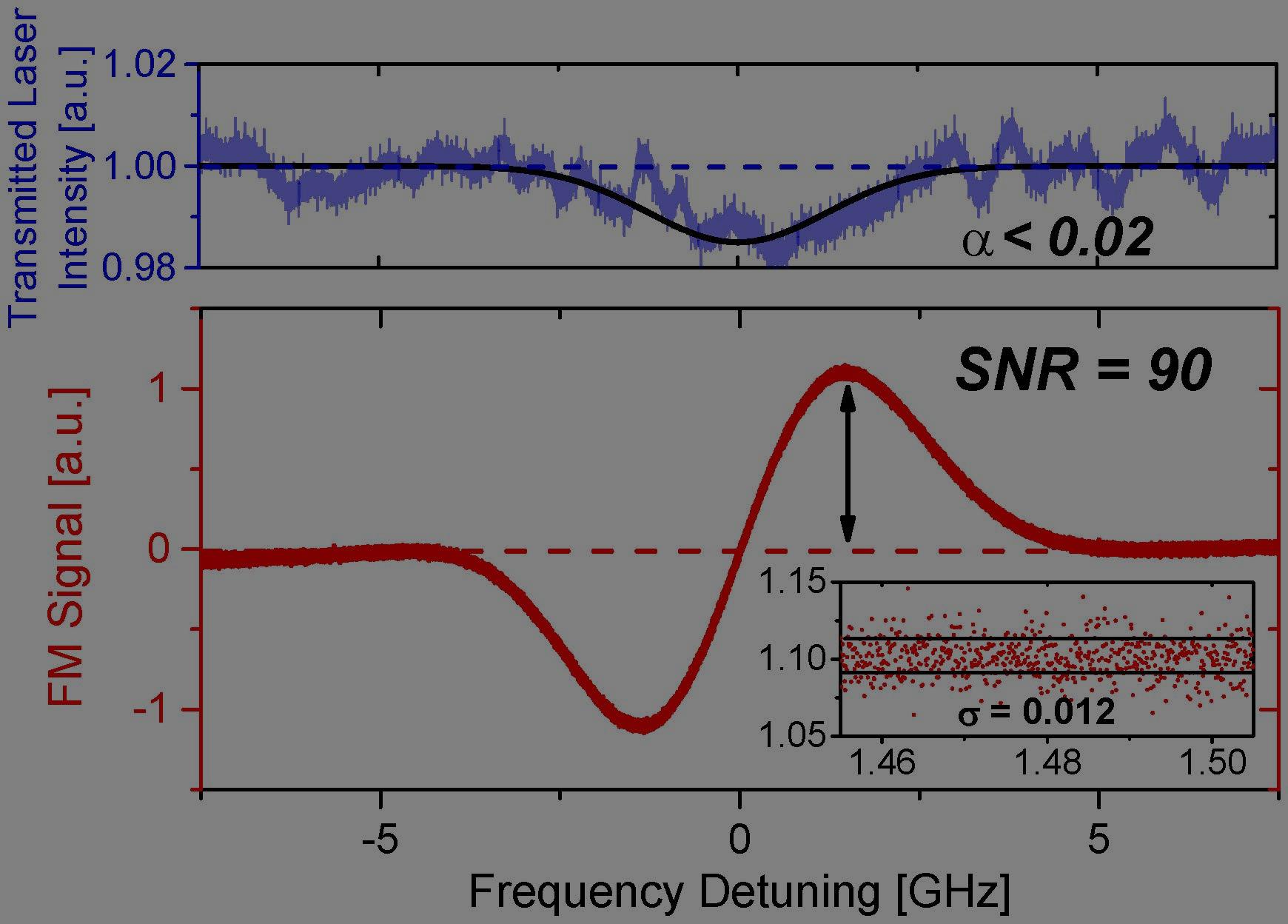
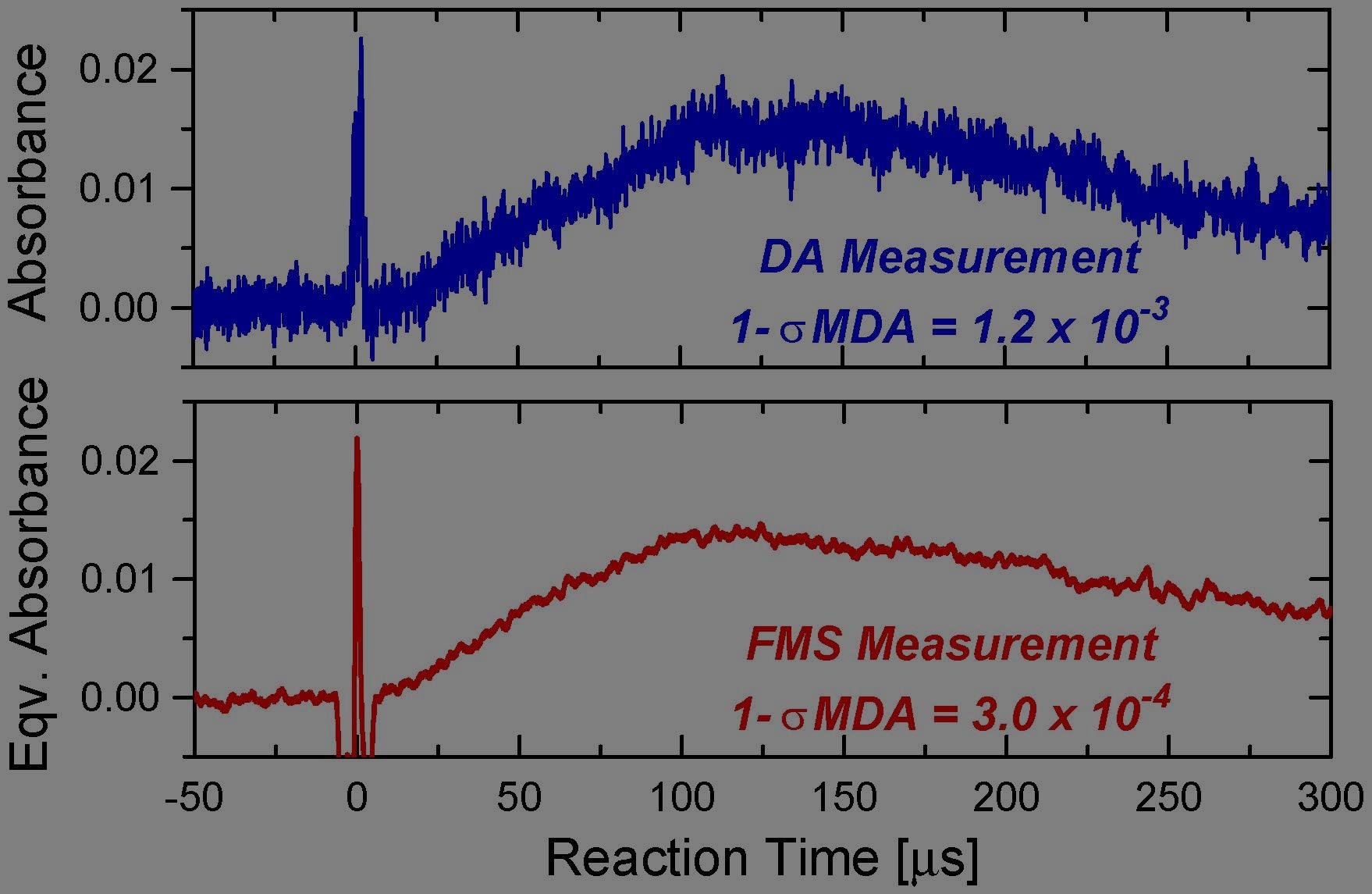
The hydroxyl (OH) radical is one of the most important transient radicals in gas-phase combustion reactions, yet it is very difficult to measure due to high reactivity, short lifetime and low concentration. Precise understanding of OH kinetics is needed for the development of novel combustion concepts and adaptation of modern engine technology to a changing fuel stream. However, significant measurement challenges have limited direct observation of OH formation/scavenging kinetics in the very early stage of fuel oxidation, during staged-ignition events, and in NTC combustion chemistry, where only minute amount of OH (as low as ppb-level) exists. Such low level of signal can easily be overwhelmed by various sources of noise, e.g. mechanical vibration, particle scattering, and fluctuation in density gradient that may cause laser beam steering, have precluded effective measurement of OH radial in these processes. In this research, an ultra-sensitive OH diagnostic method was developed for studies in high-temperature reacting gases. This method utilized an optical heterodyne scheme named Frequency-Modulation Spectroscopy (FMS), which up-shifts the signal of interest to the GHz domain far above typical noise bands and exploits lock-in amplification to extract the noise-immune signal (Fig. 3). By adapting FMS for detecting the short-lived OH radical, this work has pressed the OH detection sensitivity to the quantum/shot-noise limit. Initial demonstration of this method (Fig. 4, T = 1330 K, P = 0.38 atm, 15 cm optical path-length) has achieved an OH detection limit of 85 ppb, surpassing the previous best record by a factor of 4, and another order of magnitude improvement is anticipated at higher modulation strength and with faster detectors. This method paves a path to ppb-level OH detection capability and offers prospects to significantly advance fundamental combustion research by enabling direct study of OH kinetics during key stages of fuel oxidation that were previously inaccessible (see OSA News of July, 2018). Extension of this diagnostic to studies of other reaction systems and radical species, as well as further improvement in its sensitivity and dynamic range, is currently being explored.
References: |
|
CAVITY-ENHANCED LASER DIAGNOSTICS FOR HIGH-TEMPERATURE GASES
|
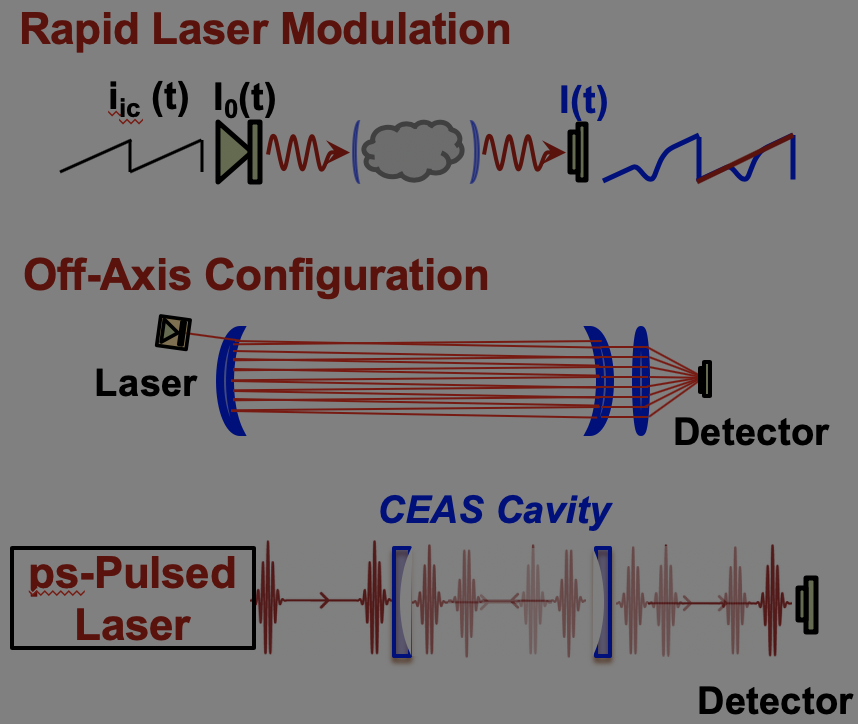
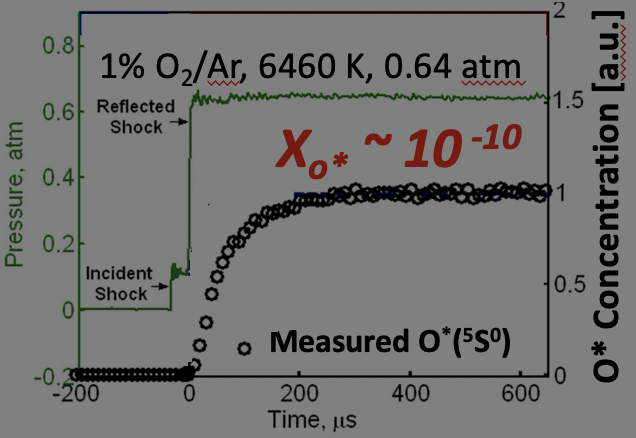
Cavity-enhanced laser spectroscopy can dramatically increase the detection sensitivities for a myriad of species over a wide wavelength range. The basic concept is to multiply the interaction pathlength between laser beam and molecules using an optical cavity, therefore overcoming the limitation in physical dimension of test facilities often encountered in the single-line-of-sight direct absorption measurement scheme. Previous applications of cavity-enhanced spectroscopy mostly focused on room-temperature and long-timescale applications, yet little was explored on measurements of high-temperature gases. The current research has advanced cavity-enhanced absorption spectroscopy to transient measurements of reacting gases behind shock waves (see Fig. 5 for representative experimental setups), and increased the detection sensitivities of several key species, such as CH3 and CO, by 20 – 140 times. This research further enables kinetic studies at ultra-dilute conditions, where significant accuracy improvement in the determination of fundamental reaction rates can be achieved due to minimization of reaction coupling. An interesting application of this method is the direct observation of electronic excitation process of atomic oxygen at extreme temperatures. This problem is important to the design of Thermal Protection System (TPS) of space vehicles during atmospheric re-entry. A space vehicle entering the lower earth orbit can reach ultra-high speed (Mach number up to 25), which creates a detached bow shock in front of the vehicle. Behind the shock wave, the air undergoes a series of strongly non-equilibrium processes including coupled vibrational relaxation and dissociation of molecular oxygen and nitrogen (O2 and N2), electronic excitation of atomic species (O and N), ionization and recombination, which turn the air into a highly-excited and partially-ionized cloud of plasma. A significant portion of its energy is transported to the surface of the vehicle via radiation, in which the excited atomic species play an important role. Of particular interest to this research was the excitation kinetics of atomic oxygen (from the ground state 3P to the first parity-allowed excited state 5S), which was studied in shock tube over 5400 – 8000 K using laser absorption. One critical challenge encountered in this study was the extremely low Boltzmann fraction of excited O resulting from the large energy gap (9 eV) between the ground and excited states. Nonetheless, by using cavity-enhanced spectroscopy, this study has successfully resolved the formation process of O(5S) (see Fig. 6) at a mole fraction level of as low as 10^-10! Based on these measurement results, this study offered the first direct determination for the rate constants of the heavy-particle collision-induced excitation of O. This work was highlighted by the OSA Spotlight on Optics in Feb. 2016. |
|
LASER ABSORPTION STUDY OF REACTION KINETICS
As important kinetic targets critically needed for the development, validation, and optimization of modern combustion theories and models, high-quality experimental data are the very bedrock on which mathematical modeling of complex gas-phasing reaction systems are anchored. However, such data are usually difficult to obtain and interpret, due particularly to the multiphysics nature of most combustion systems investigated, i.e. the strong coupling of chemical kinetics, fluid mechanics, and mass/heat transport. This issue can be further compounded by the difficulty of determining the starting time of combustion reactions.
This issued is resolved by utilizing shock waves for prompt initiation of high-temperature gas reactions, e.g. in shock tube facilities. A typical shock tube is a closed cylindrical tube comprised of a driven section and driven section that are separated by a thin diaphragm. During a shock wave experiment, the driven section is filled with the test gas mixture, and the driver section is over-pressured with inert gas, often helium or a helium mixture, until the diaphragm bursts. An incident shock wave quickly forms and travels towards the shock tube end wall, where it reflects and stagnates the gas behind, in the meantime increasing its temperature and pressure. In such a way, shock tubes can create a nearly zero-dimensional test environment with well-defined temperature, pressure, and time-zero, therefore isolating elemental reaction kinetics from the effects of gas flow and transport. This unique capability of shock tubes is exploited in conjunction with high-speed (μs-resolution) laser absorption diagnostics to provide important kinetics target data for improving modern reaction models.
A representative example is the use of shock tube and laser absorption techniques to study aldehydes reaction kinetics. Aldehydes, especially formaldehyde (CH2O), are key intermediate species found in the pyrolysis and oxidation of virtually all hydrocarbon fuels. They are generally formed during the pre-ignition stage of combustion and subsequently removed during ignition. As such, aldehydes are important benchmarks for engine operation, as abnormal amounts of remaining aldehydes usually indicate reaction quenching or incomplete combustion. Besides, aldehydes are toxic and strictly regulated by VOC emission standards, which creates an additional challenge for the adaptation of bio-derived oxygenated fuels as they tend to produce more aldehydes during their initial stage of combustion. Recent efforts of co-optimizing engines and fuels demand improved knowledge on the formation and removal kinetics of aldehydes. Using laser absorption diagnostics, the fundamental reaction rate constants of several key removal channels of aldehydes have been accurtely determined via shock tube experimentation.
Recently, an emerging trend in the reaction kinetics community is the increasing emphasis on predictive modeling. Consequently, there is a paradigm shift regarding how modern kinetic experiments should be conducted. “Randomly-sampled”, “try-and-error”, or “data-dumping” types of experiments are giving place to carefully designed and precisely executed experiments tailored to answer specific questions. The logic is quire clear: since good experiments are expensive, one would like to prioritize resources on the most informative studies first. Within the scope of the current research, an important topic under investigation is how to pin-point the conditions of laser absorption / shock tube experiments where maximum expected information gain can be achieved. A different but not entirely uncorrelated topic also being investigated is to identify and test other reaction initiation mechanisms (aside from shock waves) that allow extended range of precisely-controlled experimental conditions.
References:
S. Wang*, D. F. Davidson, R. K. Hanson, Shock tube measurements for the rate constants of long, branched, and unsaturated aldehydes with OH at elevated temperatures, Proceedings of the Combustion Institute, 36 (2017) 151-160.
S. Wang*, D. F. Davidson, R. K. Hanson, Shock tube measurement for the dissociation rate constant of acetaldehyde using sensitive CO diagnostics, Journal of Physical Chemistry A, 120 (2016) 6895-6901.
S. Wang*, D. F. Davidson, R. K. Hanson, High temperature measurements for the rate constants of C1–C4 aldehydes with OH in a shock tube. Proceedings of the Combustion Institute, 35 (2015) 473-480.
S. Wang, E. E. Dames, D. F. Davidson*, R. K. Hanson, Reaction rate constant of CH2O+ H= HCO+ H2 revisited: A combined study of direct shock tube measurement and transition state theory calculation. Journal of Physical Chemistry A, 118 (2014) 10201-10209.
S. Wang, D. F. Davidson*, R. K. Hanson, R. K. High-temperature laser absorption diagnostics for CH2O and CH3CHO and their application to shock tube kinetic studies. Combustion and Flame, 160 (2013) 1930-1938.
OTHER RESEARCH INTERESTS
The precision laser spectroscopy methods developed in the above research programs can be used for applications in other research areas as well. These areas include, for example, in situ / in operando spectroscopy of catalytic reaction kinetics, trace toxin detection and source-tracing in atmospheric and oceanic monitoring, and non-invasive biomedical diagnoses, etc. Application-specific diagnostics may be available upon request. If you have a specific measurement need or an interesting scientific/technical problem that may be solvable with laser spectroscopy, please email Shengkai directly, and he will be delighted to help.